

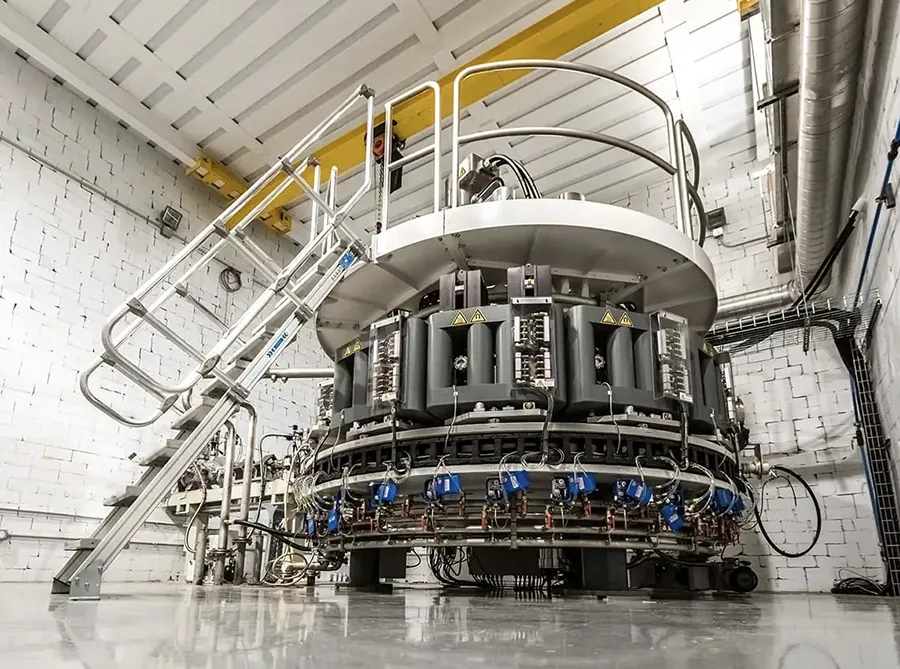
Our
electron beam
accelerators
Radioisotopes
without the use of
uranium

We’ve developed Cu-67 and Ac-225 production processes that are non-reactor based. These processes are environmentally preferred and allow for reliable, scalable, commercial quantities.

One mission
One campus
Our Mission:
Providing Patients Global Access to Game-Changing Radiopharmaceuticals
We have
the people
the technology
the facilities
all in one campus

Technologies that advance the availability of radiopharmaceuticals are increasingly critical for the treatment of cancer. NorthStar’s patient-focused Radiopharmaceutical Contract Development and Manufacturing Organization (CDMO/CMO) addresses this important need.
Increase
your knowledge
From in-depth white papers to videos and publications, feel free to delve into the insights presented here and take your journey into game-changing radiopharmaceuticals to new heights.
