Actinium-225
Actinium-225 (Ac-225) is considered one of the rarest radioisotopes on earth. It is not naturally occurring and therefore needs to be manufactured. There are several technologies used to produce Ac-225 but none that can scale to meet commercial quantities. NorthStar’s method is capable of meeting customer demand.

PRODUCING SUFFICIENT SUPPLY
TO MEET GLOBAL DEMAND
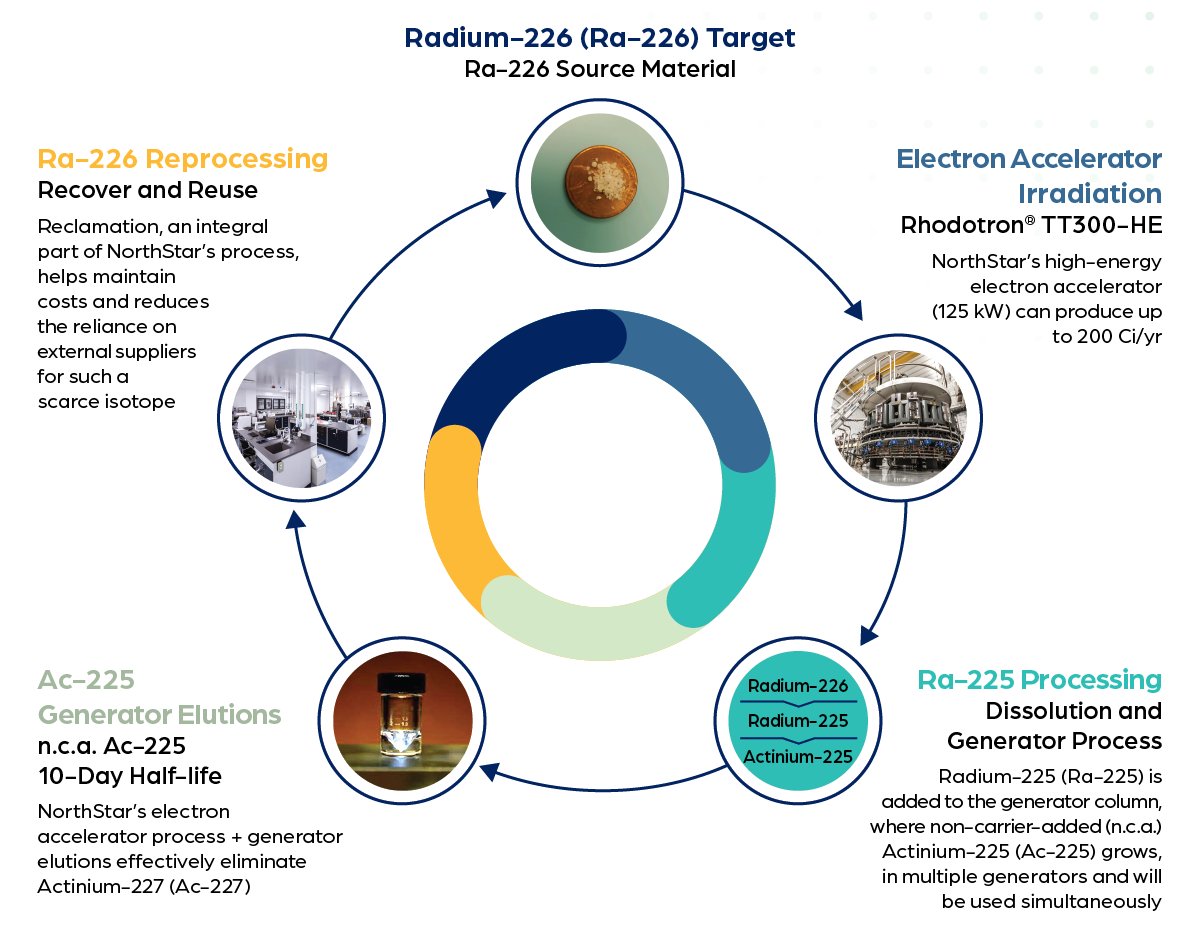
NorthStar is pioneering groundbreaking technology to produce commercial quantities of actinium-225 from radium-226 targets using electron beam accelerators. The process begins with the acceleration of an electron that collides with a converter. The converter absorbs the energy from the electrons, converting it into a high-energy photon that collides with the target material, removing a neutron from the nucleus of the atoms resulting in radium-225.
Next, a chemical purification process is completed removing all actinium, including actinium-227, leaving a purified radium solution. The purified radium solution is allowed to decay and produce non-carrier added (n.c.a.) Ac-225. This process substantially increases production capacity and flexibility due to multiple radium solutions being on-line simultaneously.